Select a different country or region to see specific content for your location.
Diagnostics
Plastic materials and parts for Diagnostics
Materials and parts for Diagnostics
If you are a medical device manufacturer or laboratory engaged in the diagnosis and treatment of illness, we have a range of products and services supporting your requirement for biologically inert materials and products. Machined parts with the most demanding level of accuracy and cleanliness:
Design and material advice
Prototype production
Demanding geometries
Bespoke quality control
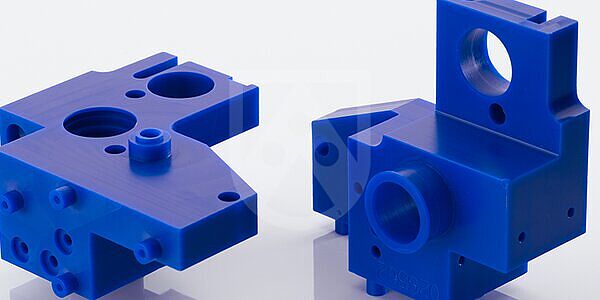
Typical parts include
Extraction spacers
Load lack chambers
Manifolds
Housings
Connectors
Plate guides
Application examples
The performance and service life of materials used in the healthcare sector are influenced by a variety of factors. When choosing the right material, factors such as surface quality requirements, contact with cleaning agents, disinfectants, dimensions and/or tolerances are essential.
Selection of Medical Grade Products
- PEEK
SustaPEEK MG black
- PEEK
SustaPEEK MG natural
- PEEK
SustaPEEK MG blue BL
- PEEK
SustaPEEK MG red RD
Selection of Healthcare Grade products
- PE > PE-HD - PE 300
Polystone® G HG white
- PVDF
SustaPVDF HG natural