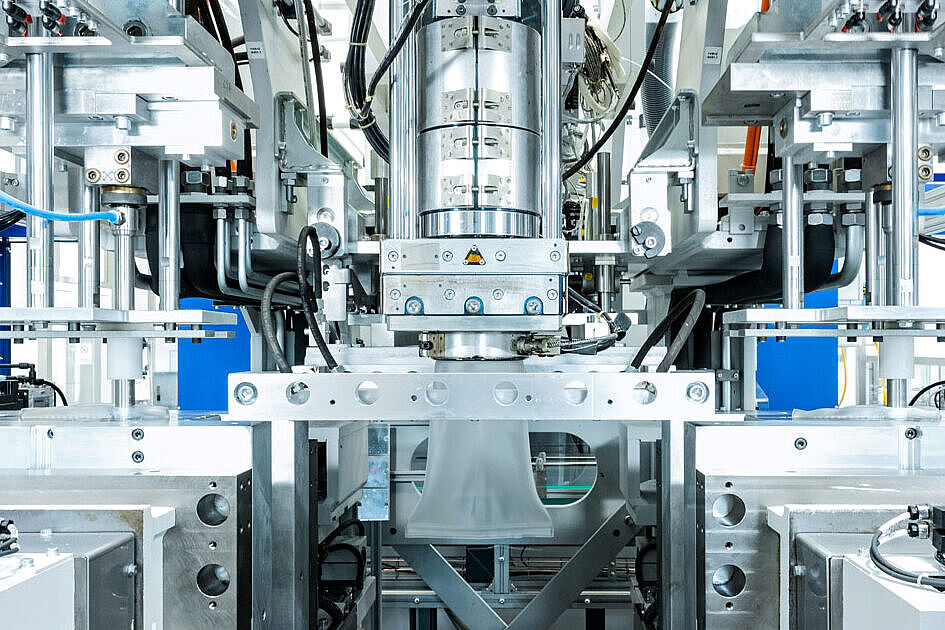
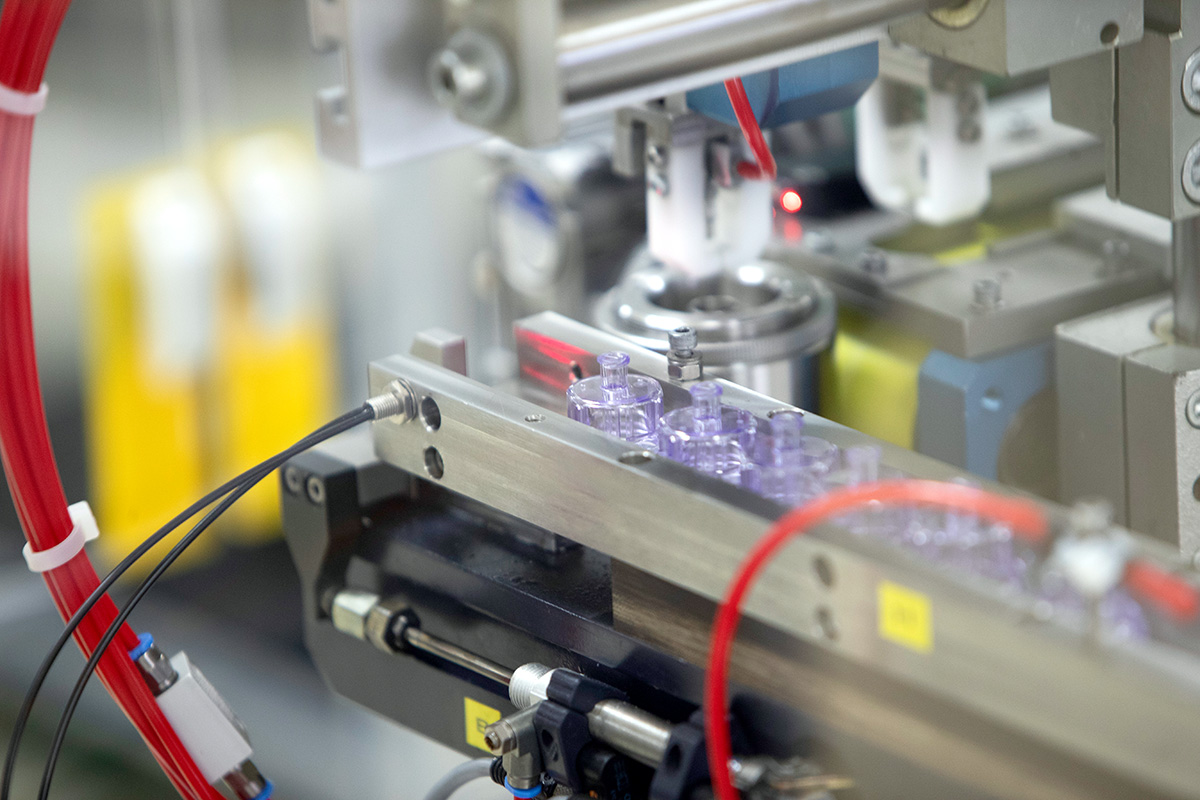
Röchling Medical Solutions Neuhaus
With over 60 years of experience in process technology and 35 years in cleanroom production, Neuhaus am Rennweg is Röchling Medical's competence center for cleanroom blow molding. Applying a comprehensive range of blow molding techniques the site produces containers, bottles and vials according to pharmaceutical packaging standard in state-of-the-art cleanrooms certified according to GMP grades D & C.
Specialists for Cleanroom Blow Molding
We manufacture and assemble:
- Pharmaceutical Primary Packaging for Solid and Liquid Dosage Forms
- Bottles and Delivery Systems for Ophthalmic Applications
- Vials for Injectables
- Drug Delivery Devices
- Consumables for Diagnostics and Analytics
Our Production Capabilities
- 7,050 m² cleanroom space certified according to GMP D & GMP C standards
- ISO 5 manufacturing capabilities
- Total 48,500 m² production and logistics area
- Cleanroom Assembly and Packaging
- In-house Packaging Development and Sterilization Coordination
Our Certifications
- ISO 15378 | Primary Packaging for Pharmaceuticals (GMP)
- ISO 13485 | Medical Devices – Quality Management Systems
- ISO 8317 | Child Resistant Packaging – Requirements and Testing Procedures for Reclosable Packages
- ISO 50001 | Energy
- ISO 14001 | Environment
- ISO 45001 | Occupational Health and Safety Management System
- ISCC Plus | Certified Materials for Circular and Bio-Based Packaging Solutions
- Drug Master Files (for selected products)
Experience Our Sister GMP-Sites in Brensbach and Neuhaus
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
If you have any questions about our competence center in Neuhaus am Rennweg, please feel free to contact us. We look forward to hearing from you.

Thierry Arnaud
Vice President - Sales & Marketing Europe

Alexander Stauch
Site Director