Sterile or Sterilized Products Safely Packaged & Delivered
We strive to optimally protect the sensitive products we manufacture during storage, transportation and delivery, ensuring seamless logistics and process chains for our customers. In-house and together with certified partners, we develop customized packaging systems and transportation concepts and coordinate and validate the overall sterilization process.
Customized Packaging & Logistics Solutions
Thanks to our in-house packaging development , we are able to offer the optimal packaging solutions to deliver pharmaceutical primary packaging or drug delivery products safely to your processing lines.
For the customized packaging of medical devices we work closely with certified partners. Our packaging concepts take into account individual sterilization requirements, transfer processes at filling or processing lines, general transportation requirements and environmental goals. We offer highly customized packaging concepts according to ISO 11607 as well as standard solutions.


Packaging Options:
- Carton packaging
- PE bags (vacuumed or non-vacuumed)
- Clean pack and brickpack packaging
- Blisters
- Trays
- Tyvek®
- Labeling
Packaging is carried out manually as well as fully automated under clean room and GMP conditions.
The Right Sterilization Solution for Your Product
To ensure the microbiological requirements of a product and thus user and patient safety, Röchling Medical has built up in-depth expertise in sterilization processes and their validation.
We take care of the entire process from developing the right packaging to verifying and validating the sterilization process and coordinating with sterilization providers. Through our network of certified partners, we can offer all common sterilization processes and select according to your requirements:
Physical:
- Gamma Irradiation
- X-ray Irradiation
- E-beam Irradation
- Autoclaving
Chemical:
- Ethylene oxide
- Plasma sterilization
Our Additional Service: Sterile, Ready-To-Fill Containers for Pharmaceuticals
ISO 11607-1:2019 specifies complex requirements for the development and validation of packaging systems for sterile products or sterile devices. At Röchling Medical in Neuhaus, we handle this process for you with our full-service offering for sterile products.
- 100% validated sterilization processes that guarantee and document an effective sterility assurance level (SAL 10-6).
- 100% validated sterile packaging systems (for sterile preservation of pharmaceutical primary packaging after sterilization, transportation, storage and aging).
- We perform microbiological monitoring for your sterile products in collaboration with qualified partners.
- Flexibility through partnerships with a range of qualified suppliers in Europe for all common sterilization processes.
- Available for a range of plastic primary packaging products, including smaller batches.



Competences at our locations
Case Studies
As a solution provider, we combine our expertise in research & product development, materials & technologies, and automation & industrialization with our experience, our quality standards, and our ambition to work with our customers to design a product that meets their individual requirements.
Our case studies give you an insight into the challenges faced by our customers in pharma, diagnostics and medical technology sectors and the tailor-made solutions that we jointly created with them.

Patient-Centric Pharmaceutical Packaging Design
Optimizing a Complex Class 3 Medical Device

Innovative Plastic Protectors for Glass Injection Vials

Custom Automation Concepts
Design for Manufacturability

Automated Quality Control

Metal and Plastic Processing from a Single Source
Medical Device Contract Manufacturing
A Single Use Medical Device Designed for Sustainability
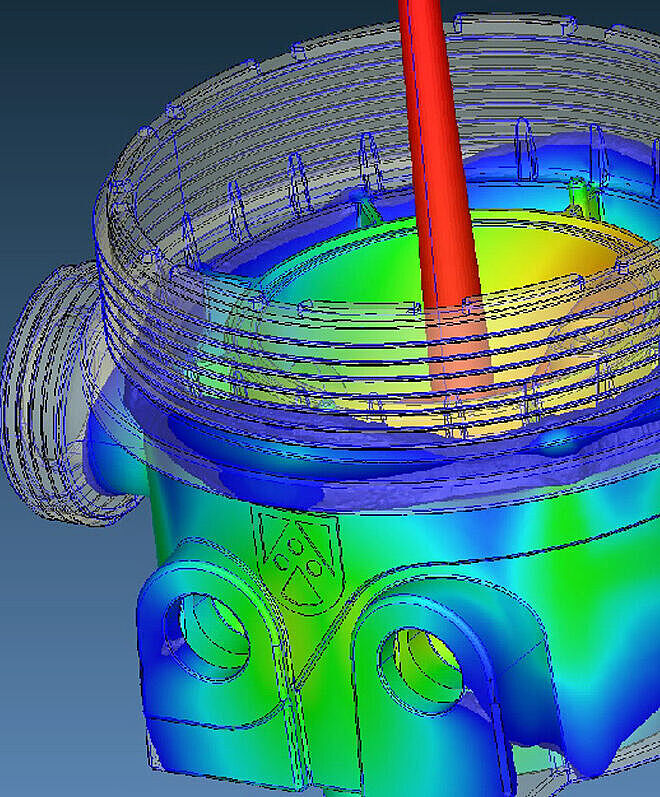
Simulations in Product Development
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical technology and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our Sterilization Services and Packaging Logistics, please contact our team.
We look forward to hearing from you.
Your Contact in Europe

Thierry Arnaud
Vice President - Sales & Marketing Europe
Your Contact in the US

Bill Ruth
Vice President - Sales & Marketing