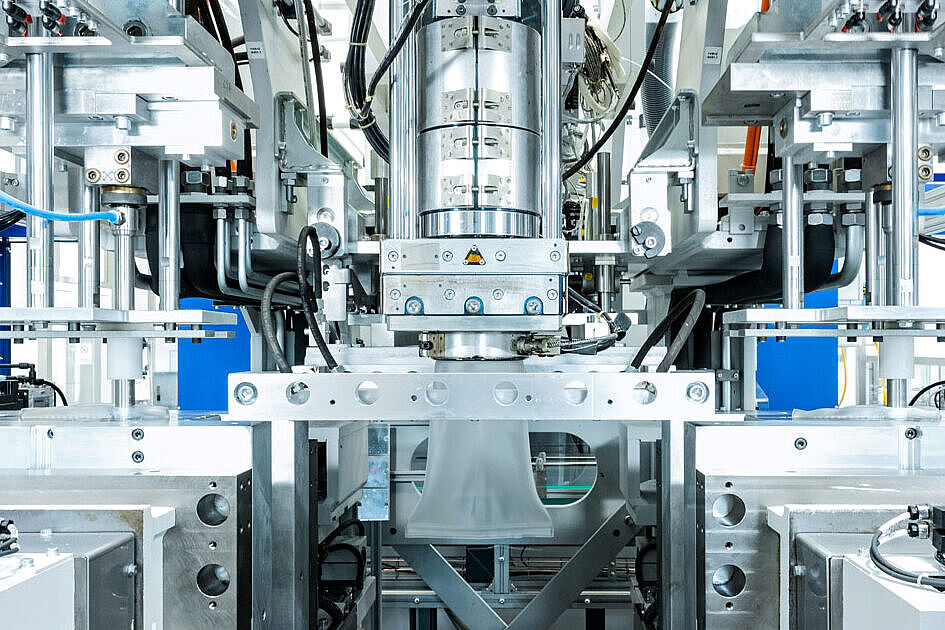
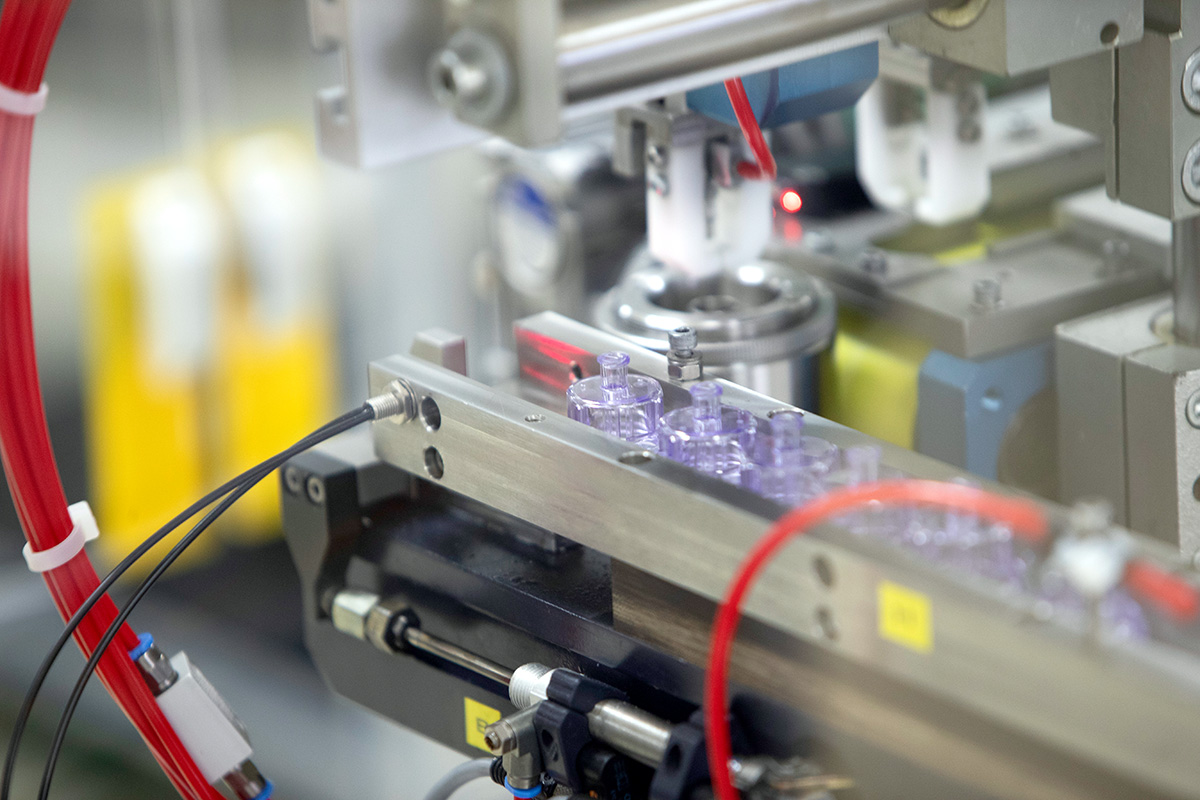
Simulation As the Key to Optimal Product Development in Medical Technology
A Carrying Role
Our customer asked us for support in the development and production of a housing system for an electronic medical device used in the emergency services for life-saving treatment.
Housing systems serve to protect a sensitive and high-quality medical device and at the same time function as a transport and application aid due to the seamlessly integrated carrying handle. For this reason, stability and break resistance are essential at all times. Additional requirements, such as complex geometries, widely varying wall thicknesses in the component and tight tolerances, challenged us during product development.
The Challenge:
- Robust housing to protect a high-quality medical device with carrying function
- Application-specific requirements for complex geometries, varying wall thicknesses and tight tolerances
- Guaranteed stability and break resistance
Error Avoidance in the Development of Medical Devices Through Simulations
Our in-house development team of designers, engineers and ergonomists combines innovative strength with many years of industry experience and established engineering knowledge. This benefits us in the design of components and tools suitable for plastics in order to ensure that, in addition to other individual principles, we always operate in accordance with the Design for Manufacturing approach.
Our versatile CAE solutions enable us to perform simulations on components and tools with the aid of intelligent software in order to identify and avoid possible sources of error from the outset and to develop an optimum design concept adapted to customer and application requirements. After initial concept ideas, we performed several injection molding simulations for our customer with different variations of number and positioning of gating points, cold and hot runner variants and cooling circuits to simulate the best gating situation for flawless results.
CAE Solutions That Pay Off
By combining our broad range of CAE solutions with our experienced industry knowledge in product development for medical technology, we are able to use a wide variety of simulations profitably for our customers:
- Fluid mechanics simulations (CFD) for the optimization of components and tools by simulating the flow behavior and checking sources of error such as dead zones, vorticity, pressure drop, and many others.
- Structural mechanics simulations (FEM) for the optimization of components and thermal analysis by testing stress and strain distribution, deformation behavior and reaction forces
- Injection molding process simulations to optimize components and contours by testing filling behavior, welding lines, air inclusions, deformations and more
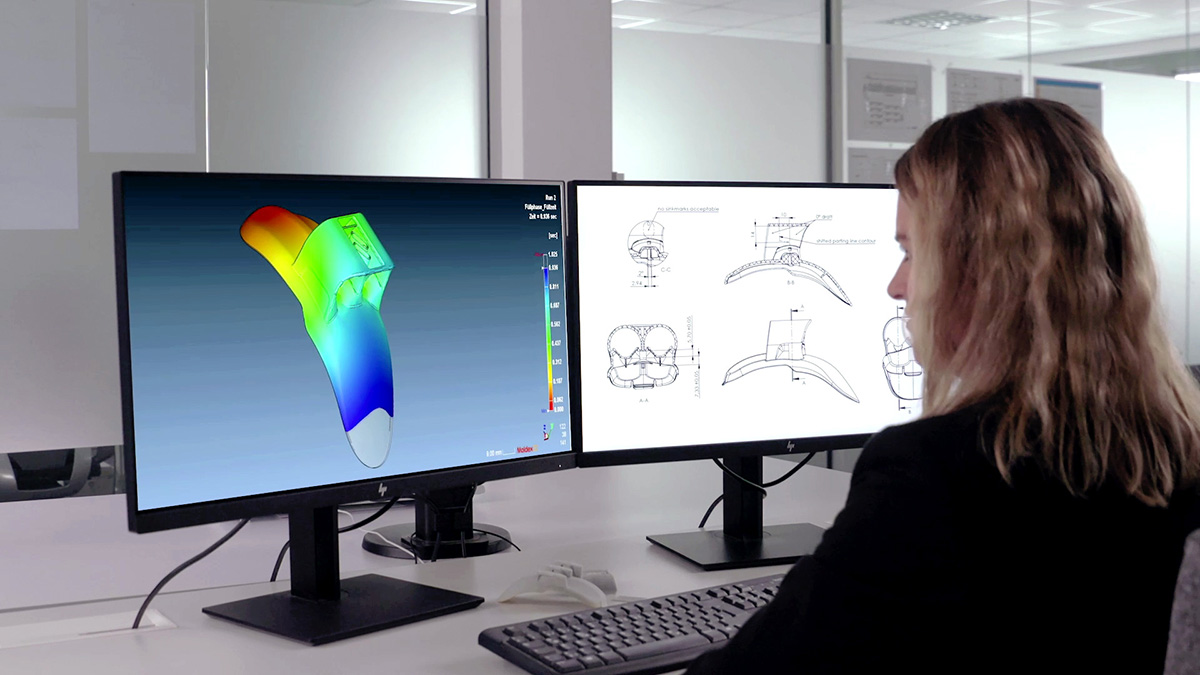
Significant Time and Cost Savings in the Product Development Process
Based on the results of the simulations, we were able to provide our customer with recommendations for further optimization of the product design as well as an ideal concept for the component design and gating system.
In addition to the smartly selected parameters such as gating points, runners and runner systems, intelligent functions like cascade control were used to ensure flawless part quality and to meet all requirements for shape, dimensions and tolerances. After the customer's approval, this concept was turned into reality, the mold was built and the first parts were produced.
The Result
The new concept made it possible to avoid some weld lines and to place the weld lines, which are unavoidable in injection molding, in a controlled manner in places where they do not compromise quality and stability. Thanks to the simulations carried out in advance, the sampling loops required before series production were halved and the total time required between mold construction and series production was minimized to one third.
In addition to the time aspect, this also means a considerable cost advantage for the customer, since the costs of physical changes to the tool are up to five times more expensive than necessary design changes, which can be identified and implemented in advance through simulations.
This video serves as an example.
To Summarize:
- Time required between tool construction and series production reduced to one third compared to empirical values
- Costs incurred for design changes up to five times lower
- Higher quality and process reliability due to previously discovered and avoided sources of error
- More sustainable production due to fewer physical changes to the tool
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our competences in product design and development, please contact our team.
We look forward to hearing from you.
Your Contact

Thierry Arnaud
Vice President - Sales & Marketing Europe