Röchling Medical Lancaster
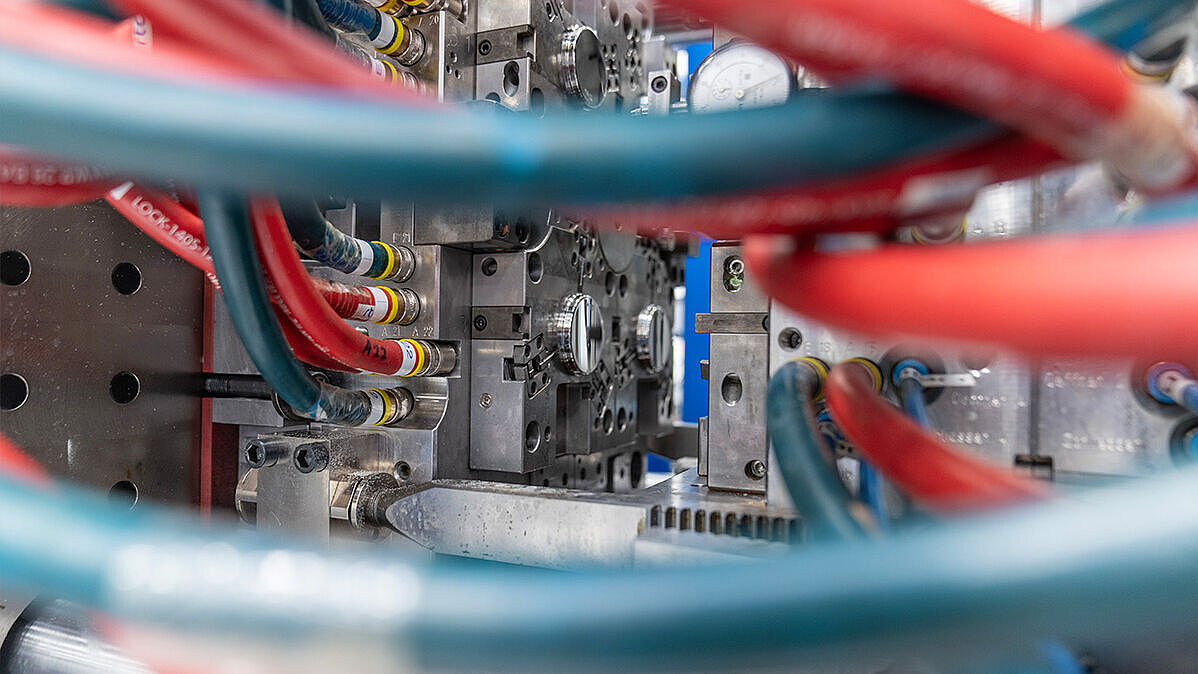
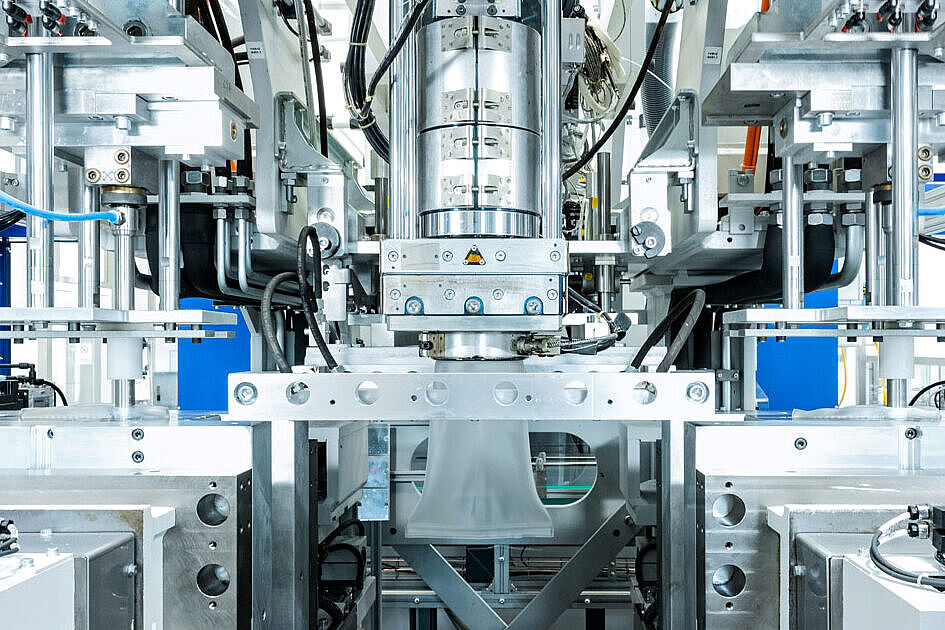
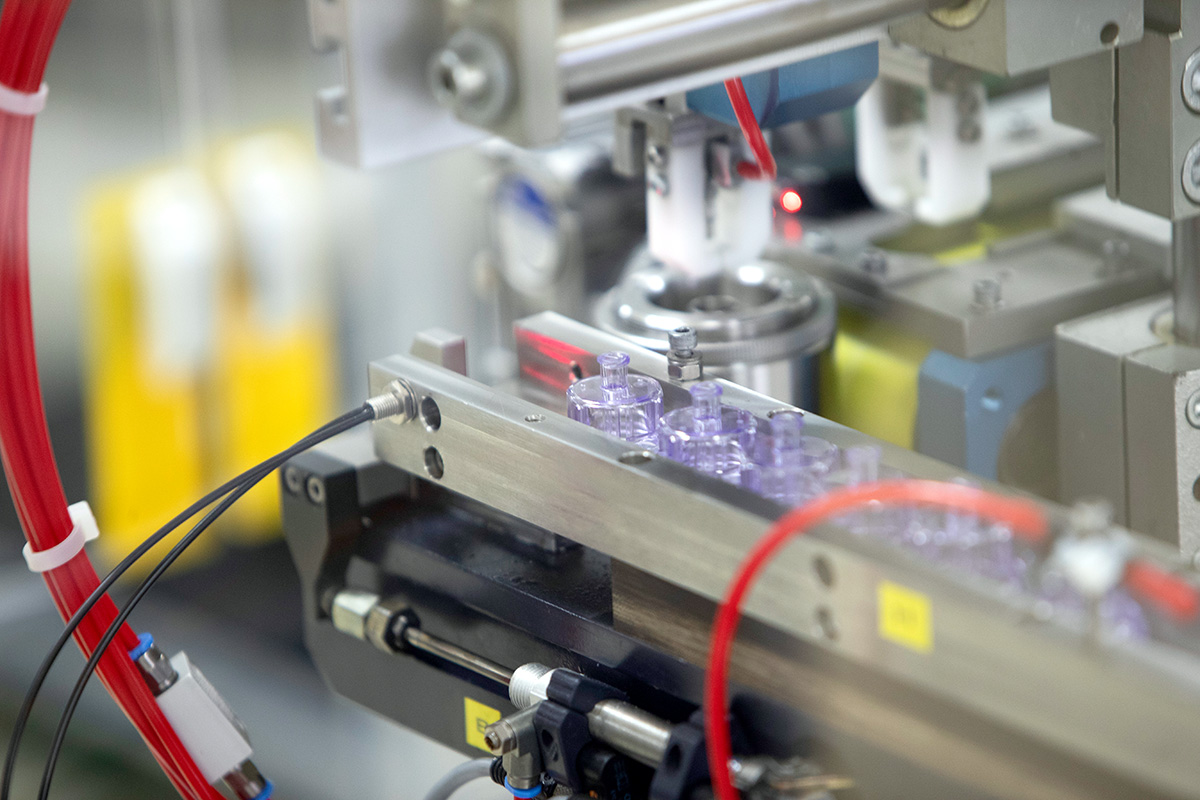
Since its inception in 1997, Röchling Medical Lancaster has focused on being a vertically integrated contract manufacturer for the medical device industry. By integrating metal and plastic processing technologies within a state-of-the-art facility, Röchling Medical Lancaster can simplify customers' supply chains, improve process flow, and manufacture devices more cost effectively.
Full-Service Provider for Medical Device with Extensive Technical Expertise
Medical device areas of expertise:
- Bio-Defense Related Products
- Orthopedics – spinal implants and bone pastes
- Endoscopy – hand held devices (flexible and rigid)
- Ophthalmology – eye surgery devices
- General Surgery
- Diabetic Delivery Systems
- Drug Delivery Systems, e.g. pen injector devices
- Diagnostics – In Vitro and POC
- Cardiovascular
- Dental
- Sterile Bifurcated Needles (smallpox/ mpox vaccination and allergy testing)
Our Production Capabilities
- Wide array of process capabilities to transform both plastic and metal materials, including titanium
- Cleanrooms certified according class 7 / class 8, ISO 14644-1
- 14 injection molding machines ranging from 20 to 180 tons
- Insert molding and micromolding
- Complex device assembly and processing
- Contract packaging (Medical Device/Pharma)
Our Certifications
- ISO 13485 | Medical Devices – Quality Management Systems
- FDA registered site
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
If you have any questions about our competence center in Lancaster, please feel free to contact us.
We look forward to hearing from you.

Bill Ruth
Vice President - Sales & Marketing

Brian Tennies
General Manager